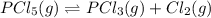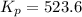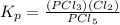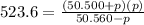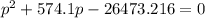Chemistry, 29.07.2019 17:30, davestrider404
For the reaction pcl5(g) 4 pcl3(g) 1 cl2(g) kp 5 23.6 at 500 k a. calculate the equilibrium partial pressures of the reactants and products at 500 k if the initial pressures are ppcl5 5 0.560 atm and ppcl3 5 0.500 atm.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, NorbxrtThaG
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 22.06.2019 22:30, jkjjoijjm5928
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 08:10, 20dyeaubn
Time remaining 58: 10 an atom that has 84 protons and 86 neutrons undergoes a reaction. at the end of the reaction, it has 82 protons and 84 neutrons. what happened to the atom? it accepted radiation in a chemical reaction it donated neutrons to another atom in a chemical reaction it emitted an alpha particle in a nuclear reaction. it accepted protons in a nuclear reaction. mark this and retum save and exit next submit
Answers: 3
Do you know the correct answer?
For the reaction pcl5(g) 4 pcl3(g) 1 cl2(g) kp 5 23.6 at 500 k a. calculate the equilibrium partial...
Questions in other subjects:


Mathematics, 18.09.2019 19:20

Social Studies, 18.09.2019 19:20


Social Studies, 18.09.2019 19:20

Social Studies, 18.09.2019 19:20

Mathematics, 18.09.2019 19:20



Biology, 18.09.2019 19:20


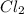 = 42.9 atm,
= 42.9 atm, 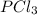 = 93.4 atm and
= 93.4 atm and 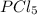 = 7.66 atm
= 7.66 atm