
Chemistry, 27.07.2019 00:30, moisealafleur
For the reaction n2o4(g) m 2 no2(g), a reaction mixture at a certain temperature initially contains both n2o4 and no2 in their standard states (see the defi nition of standard state in section 6.9 ) . if kp = 0.15, which statement is true of the reaction mixture before any reaction occurs? (a) q = k; the reaction is at equilibrium. (b) q 6 k; the reaction will proceed to the right. (c) q 7 k; the reaction will proceed to the left.

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:30, Tooey2331
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 18:30, sarahbug56
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
Do you know the correct answer?
For the reaction n2o4(g) m 2 no2(g), a reaction mixture at a certain temperature initially contains...
Questions in other subjects:


Mathematics, 23.05.2020 21:58









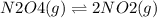
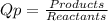
![Qp = \frac{[NO2]^{2} }{[N2O4]} \\\\Under\ standard\ state\ : \\\\Pressure\ NO2 = \ Pressure\ N2O4\ = 1\ atm\\\\Qp = \frac{[1.00]^{2} }{[1.00]} = 1](/tpl/images/0136/8396/d2f96.png)





