Calculate the net change in energy for the following reaction:
2na(s) + 2hcl(g) → 2nacl(s) +...

Chemistry, 26.07.2019 16:20, flowersthomas1969
Calculate the net change in energy for the following reaction:
2na(s) + 2hcl(g) → 2nacl(s) + h2(g)
given the following information:
energy of sublimation of na(s) = 97 kj/mol
bond energy of hcl = 427 kj/mol
ionization energy of na(g) = 496 kj/mol
electron affinity of cl(g) = –349 kj/mol
lattice energy of nacl(s) = –778 kj/mol
bond energy of h2 = 432 kj/mol

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 03:50, KAITLYN007
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1

Do you know the correct answer?
Questions in other subjects:







Mathematics, 13.03.2020 00:59

Mathematics, 13.03.2020 00:59



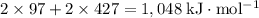 .
.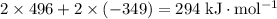 .
.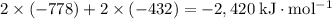 .
.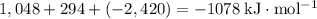 .
.





