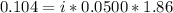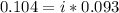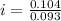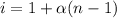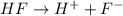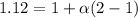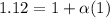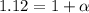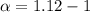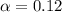Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:10, ChloeLiz7111
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 03:00, actheorian8142
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 10:30, kylemartinez13
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
Do you know the correct answer?
Asolution is made by dissolving 0.0500 mol of hf in 1.00 kg of water. the solution was found to free...
Questions in other subjects:


Social Studies, 09.10.2019 20:30

Chemistry, 09.10.2019 20:30

History, 09.10.2019 20:30

World Languages, 09.10.2019 20:30

History, 09.10.2019 20:30



Mathematics, 09.10.2019 20:30


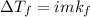
 =0.104 degree C
=0.104 degree C