

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1


Chemistry, 22.06.2019 00:30, jamesnaquan132
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 07:30, bbyniah123
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3
Do you know the correct answer?
Identify the brønsted-lowry acid, the brønsted-lowry base, the conjugate acid, and the conjugate bas...
Questions in other subjects:



History, 11.10.2019 06:30


English, 11.10.2019 06:30

Physics, 11.10.2019 06:30

Mathematics, 11.10.2019 06:30


English, 11.10.2019 06:30


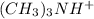 and
and 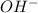 are conjugate acid and base respectively.
are conjugate acid and base respectively.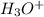 and
and 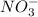 are conjugate acid and base respectively.
are conjugate acid and base respectively.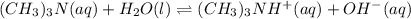
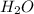 is an acid that donate a proton or hydrogen to
is an acid that donate a proton or hydrogen to 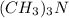 base and it forms
base and it forms 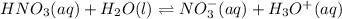
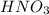 is an acid that donate a proton or hydrogen to
is an acid that donate a proton or hydrogen to 



