
In the first step of the ostwald process for the synthesis of nitric acid, ammonia is converted to nitric oxide by the high-temperature reaction 4 nh31g2 + 5 o21g2 ¡ 4 no1g2 + 6 h2o1g2 (a) how is the rate of consumption of o2 related to the rate of consumption of nh3? (b) how are the rates of formation of no and h2o related to the rate of consumption of nh3

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
Do you know the correct answer?
In the first step of the ostwald process for the synthesis of nitric acid, ammonia is converted to n...
Questions in other subjects:


Social Studies, 21.03.2020 10:02

Mathematics, 21.03.2020 10:02


Mathematics, 21.03.2020 10:02


Mathematics, 21.03.2020 10:02

Mathematics, 21.03.2020 10:02

Mathematics, 21.03.2020 10:02


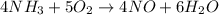
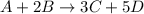
![rate=-\frac{\Delta [A]}{\Delta t}=-\frac{1}{2}\frac{\Delta [B]}{\Delta t}=\frac{1}{3}\frac{\Delta [C]}{\Delta t}=\frac{1}{5}\frac{\Delta [D]}{\Delta t}](/tpl/images/0133/4682/3644a.png)
![rate=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}=-\frac{1}{5}\frac{\Delta [O_2]}{\Delta t}=\frac{1}{4}\frac{\Delta [NO]}{\Delta t}=\frac{1}{6}\frac{\Delta [H_2O]}{\Delta t}](/tpl/images/0133/4682/51a20.png)
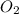 related to rate of consumption of
related to rate of consumption of 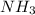 as:
as:![-\frac{1}{5}\frac{\Delta [O_2]}{\Delta t}=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/1a857.png)
![\frac{\Delta [O_2]}{\Delta t}=\frac{5}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/ce2db.png)
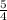 the rate of consumption of ammonia.
the rate of consumption of ammonia.![\frac{1}{4}\frac{\Delta [NO]}{\Delta t}=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/91a55.png)
![\frac{\Delta [NO]}{\Delta t}=-\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/c5068.png)
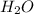 to the rate of consumption of ammonia would be:
to the rate of consumption of ammonia would be:![\frac{1}{6}\frac{\Delta [H_2O]}{\Delta t}=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/41d66.png)
![\frac{\Delta [H_2O]}{\Delta t}=-\frac{6}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/66644.png)
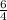 times that is 1.5 times to the rate of consumption of ammonia.
times that is 1.5 times to the rate of consumption of ammonia. 




