

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, fgcherubin
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1

Chemistry, 23.06.2019 03:50, arimarieestrada
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3

Chemistry, 23.06.2019 05:00, cpcoolestkid4
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius. a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit. a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius. a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1
Do you know the correct answer?
For the reaction below, kp 5 1.16 at 800.8c. caco3(s) 34 cao(s) 1 co2(g) if a 20.0-g sample of caco3...
Questions in other subjects:

Mathematics, 20.04.2021 21:10


Mathematics, 20.04.2021 21:10

Computers and Technology, 20.04.2021 21:10


History, 20.04.2021 21:10

History, 20.04.2021 21:10

Engineering, 20.04.2021 21:10


English, 20.04.2021 21:10


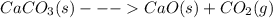
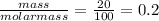
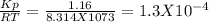
![\frac{[CO_{2}][CaO]}{[CaCO_{3}]}= \frac{x^{2} }{(0.2-x)}=1.3X10^{-4}](/tpl/images/0133/4658/99790.png)
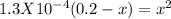
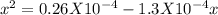
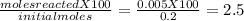 %
%



