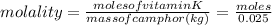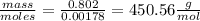Chemistry, 25.07.2019 21:20, Natasha019
Vitamin k is involved in normal blood clotting. when 0.802 g of vitamin k is dissolved in 25.0 g of camphor, the freezing point of the solution is lowered by 2.69 °c. the freezing point and kf constant for camphor can be found here. calculate the molar mass of vitamin k.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 21:00, nsutton9985
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1
Do you know the correct answer?
Vitamin k is involved in normal blood clotting. when 0.802 g of vitamin k is dissolved in 25.0 g of...
Questions in other subjects:

Biology, 29.07.2019 16:00

Biology, 29.07.2019 16:00