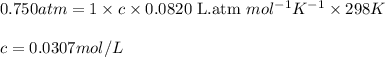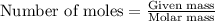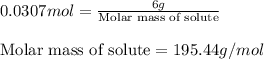Chemistry, 25.07.2019 03:10, alexiaalfaro
Asolution is prepared by dissolving 6.00 g of an unknown nonelectrolyte in enough water to make 1.00 l of solution. the osmotic pressure of this solution is 0.750 atm at 25.0°c. what is the molecular weight (g/mol) of the unknown solute? g

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1


Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
Do you know the correct answer?
Asolution is prepared by dissolving 6.00 g of an unknown nonelectrolyte in enough water to make 1.00...
Questions in other subjects:

Mathematics, 04.10.2021 14:50






Mathematics, 04.10.2021 14:50


Mathematics, 04.10.2021 15:00

Biology, 04.10.2021 15:00


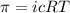
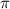 = osmotic pressure of the solution = 0.750 atm
= osmotic pressure of the solution = 0.750 atm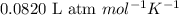
![25^oC=[273+25]=298K](/tpl/images/0129/6185/6a9f9.png)