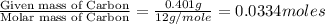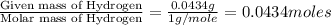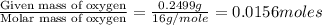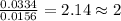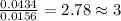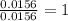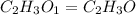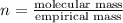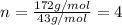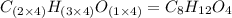Chemistry, 25.07.2019 03:10, paytonpaige22
When 0.6943 g of a compound is subjected to combustion analysis it produced 1.471 g co2 and 0.391 g h2o. what is its empirical and molecular formula if its molar mass is 172 g/mol if the compound is composed of only carbon, hydrogen, and oxygen.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, sophiebeardsley94
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

Chemistry, 21.06.2019 22:30, monnn91351
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Do you know the correct answer?
When 0.6943 g of a compound is subjected to combustion analysis it produced 1.471 g co2 and 0.391 g...
Questions in other subjects:



Mathematics, 23.02.2021 02:50


Biology, 23.02.2021 02:50

Business, 23.02.2021 02:50

English, 23.02.2021 02:50


Mathematics, 23.02.2021 02:50

English, 23.02.2021 02:50


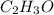 and
and 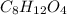 respectively.
respectively.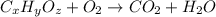
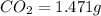
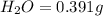
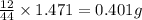 of carbon will be contained.
of carbon will be contained.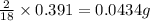 of hydrogen will be contained.
of hydrogen will be contained.