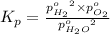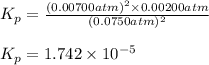Chemistry, 25.07.2019 01:20, jaymee2904p88tgh
The elementary reaction 2h2o(g)↽−−⇀2h2(g)+o2(g) proceeds at a certain temperature until the partial pressures of h2o, h2, and o2 reach 0.0750 atm, 0.00700 atm, and 0.00200 atm, respectively. what is the value of the equilibrium constant at this temperature?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, only1cache
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 10:40, justicejesusfreak
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1


Chemistry, 22.06.2019 19:20, johnkings140
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
Do you know the correct answer?
The elementary reaction 2h2o(g)↽−−⇀2h2(g)+o2(g) proceeds at a certain temperature until the partial...
Questions in other subjects:

Mathematics, 24.01.2020 03:31

Computers and Technology, 24.01.2020 03:31


Mathematics, 24.01.2020 03:31


English, 24.01.2020 03:31



Mathematics, 24.01.2020 03:31

Mathematics, 24.01.2020 03:31


 is the value of the equilibrium constant at this temperature.
is the value of the equilibrium constant at this temperature.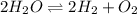

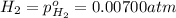
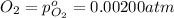
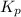 for the given chemical equation is:
for the given chemical equation is: