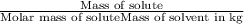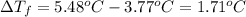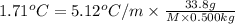Chemistry, 25.07.2019 00:30, allstar976
When a 33.8-g sample of an unknown compound is dissolved in 500. g of benzene, the freezing point of the resulting solution is 3.77°c. the freezing point of pure benzene is 5.48°c, and kf for benzene is 5.12°c/m. calculate the molar mass of the unknown compound.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:30, rosie20052019
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 23.06.2019 03:00, rayne40
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
Do you know the correct answer?
When a 33.8-g sample of an unknown compound is dissolved in 500. g of benzene, the freezing point of...
Questions in other subjects:


Chemistry, 22.11.2020 01:30

Computers and Technology, 22.11.2020 01:30



Law, 22.11.2020 01:30


Computers and Technology, 22.11.2020 01:30

Mathematics, 22.11.2020 01:30

Chemistry, 22.11.2020 01:30


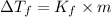
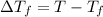
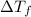 = change in boiling point = 0.81 K
= change in boiling point = 0.81 K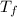 =Boiling point of the solution = 3.77°C
=Boiling point of the solution = 3.77°C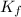 = freezing point constant = 5.12°C/m
= freezing point constant = 5.12°C/m