
Chemistry, 24.07.2019 23:20, cornpops4037
325mgofacetylsalicylic acid (hc9h7o4). calculate the ph of a solution that is prepared by dissolving two aspirin tablets in one cup (237 ml) of solution. assume the aspirin tablets are pure acetylsalicylic acid, ka 5 3.3 3 1024.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 13:10, dookiefadep5n1tt
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 20:10, maddie1776
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
Do you know the correct answer?
325mgofacetylsalicylic acid (hc9h7o4). calculate the ph of a solution that is prepared by dissolving...
Questions in other subjects:

Mathematics, 23.04.2021 20:00




Mathematics, 23.04.2021 20:00



Business, 23.04.2021 20:00

Mathematics, 23.04.2021 20:00

English, 23.04.2021 20:00


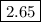
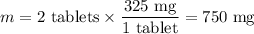
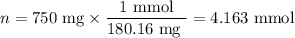
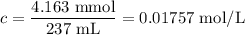
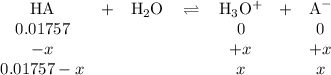
![K_{\text{a}} = \dfrac{\text{[H}_{3}\text{O}^{+}]\text{A}^{-}]} {\text{[HA]}} = 3.33 \times 10^{-4}\\\\\dfrac{x^{2}}{0.01757 - x} = 3.33 \times 10^{-4}\\\\\textbf{Check that }\mathbf{x \ll 0.01757}\\\\\dfrac{ 0.01757 }{3.33 \times 10^{-4}} = 53 < 400\\\\\text{The ratio is less than 400. We must solve a quadratic equation.}\\\\x^{2} = 3.33 \times 10^{-4}(0.01757 - x) \\\\x^{2} = 5.851 \times 10^{-6} - 3.33 \times 10^{-4}x\\\\x^{2} + 3.33 \times 10^{-4}x - 5.851 \times 10^{-6} = 0](/tpl/images/0128/9878/cedfd.png)
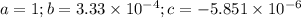
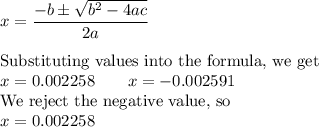
![\rm [H_{3}O^{+}]= x \, mol \cdot L^{-1} = 0.002258 \, mol \cdot L^{-1}\\\text{pH} = -\log{\rm[H_{3}O^{+}]} = -\log{0.002258} = \mathbf{2.65}\\\text{The pH of the solution is } \boxed{\textbf{2.65}}](/tpl/images/0128/9878/21391.png)




