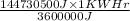Chemistry, 24.07.2019 20:10, colochaortiz20p7cajw
The most useful ore of aluminum is bauxite, in which al is present as hydrated oxides, al2o3⋅xh2o the number of kilowatt-hours of electricity required to produce 3.00kg of aluminum from electrolysis of compounds from bauxite is when the applied emf is 4.50v.

Answers: 3
Other questions on the subject: Chemistry



Do you know the correct answer?
The most useful ore of aluminum is bauxite, in which al is present as hydrated oxides, al2o3⋅xh2o th...
Questions in other subjects:

History, 15.01.2020 20:31


Biology, 15.01.2020 20:31



Mathematics, 15.01.2020 20:31

Mathematics, 15.01.2020 20:31



Physics, 15.01.2020 20:31


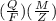
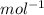
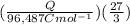
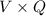
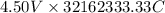
 J = 1 KW Hr
J = 1 KW Hr