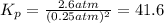Chemistry, 24.07.2019 17:10, devilrao6742
The reaction x 2 (g) m 2 x(g) occurs in a closed reaction vessel at constant volume and temperature. initially, the vessel contains only x 2 at a pressure of 1.55 atm. after the reaction reaches equilibrium, the total pressure is 2.85 atm. what is the value of the equilibrium constant, kp , for the reaction?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, torigirl4126
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
Do you know the correct answer?
The reaction x 2 (g) m 2 x(g) occurs in a closed reaction vessel at constant volume and temperature....
Questions in other subjects:




Arts, 14.11.2020 23:40

Health, 14.11.2020 23:40


English, 14.11.2020 23:40


Arts, 14.11.2020 23:40

Mathematics, 14.11.2020 23:40


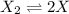
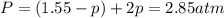
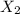 at equilibrium:
at equilibrium:![[p_{X_2}^o]=2p=2\time 1.3 atm=2.6 atm](/tpl/images/0128/0119/e4252.png)
![[p_{X}^{o}]=1.55 atm -1.3 atm = 0.25 atm](/tpl/images/0128/0119/f5d78.png)
![K_p=\frac{[p_{X_2}^o]}{[p_{X}^{o}]^2}](/tpl/images/0128/0119/aa6e2.png)