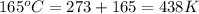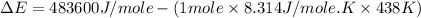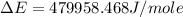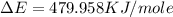Chemistry, 24.07.2019 11:20, brookeanne723
Consider the reaction 2h2o(g) → 2h2(g) + o2(g)δh = 483.6 kj/mol. if 2.0 moles of h2o(g) are converted to h2(g) and o2(g) against a pressure of 1.0 atm at 165°c, what is δu for this reaction?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 15:20, shanyeah
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1


Chemistry, 22.06.2019 22:40, lindseyklewis1p56uvi
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization. a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution. part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
Do you know the correct answer?
Consider the reaction 2h2o(g) → 2h2(g) + o2(g)δh = 483.6 kj/mol. if 2.0 moles of h2o(g) are converte...
Questions in other subjects:

Mathematics, 20.02.2021 18:20


Mathematics, 20.02.2021 18:20


Business, 20.02.2021 18:20


Mathematics, 20.02.2021 18:20

Physics, 20.02.2021 18:20

Spanish, 20.02.2021 18:20

Mathematics, 20.02.2021 18:20


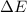 of the reaction is, 479.958 KJ/mole
of the reaction is, 479.958 KJ/mole
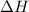 = enthalpy of the reaction = 483.6 KJ/mole = 483600 J/mole
= enthalpy of the reaction = 483.6 KJ/mole = 483600 J/mole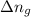 = change in the moles of the reaction = Moles of product - Moles of reactant = 3 - 2 = 1 mole
= change in the moles of the reaction = Moles of product - Moles of reactant = 3 - 2 = 1 mole