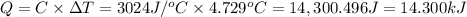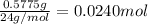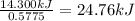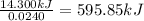A0.5775−g sample of solid magnesium is burned in a constant-volume bomb calorimeter that has a heat capacity of 3024 j/°c. the temperature increases by 4.729°c. (a) calculate the heat associated with the burning mg in kj/g. kj/g (b) calculate the heat associated with the burning of mg in kj/mol. kj/mol

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, parisaidan366
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1

Chemistry, 23.06.2019 09:30, tramqpham25
People who practice which of the following diets may run the risk of not getting enough iron. a. gluten free or vegan diet b. diet for managing diabetes c. vegan diet d. gluten free diet
Answers: 2

Chemistry, 23.06.2019 10:00, Jennifer16253
What is the mass in grams of 12.26 ml of acetone
Answers: 1
Do you know the correct answer?
A0.5775−g sample of solid magnesium is burned in a constant-volume bomb calorimeter that has a heat...
Questions in other subjects:

Mathematics, 03.02.2021 18:20

Physics, 03.02.2021 18:20

Mathematics, 03.02.2021 18:20

English, 03.02.2021 18:20




Mathematics, 03.02.2021 18:20