

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, robert7248
What is the cellular process that releases the energy stored in food molecules
Answers: 3

Chemistry, 22.06.2019 00:00, lilyclairehutson
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
Do you know the correct answer?
Where the oxygen comes from the air (21% o2 and 79% n2). if oxygen is fed from air in excess of the...
Questions in other subjects:

Social Studies, 28.06.2021 18:20

Mathematics, 28.06.2021 18:20

English, 28.06.2021 18:20




Mathematics, 28.06.2021 18:20




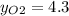 %
%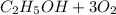 →
→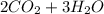
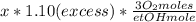
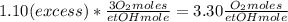
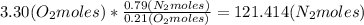
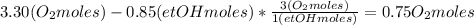
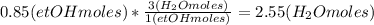
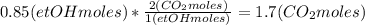
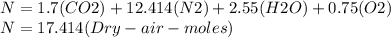
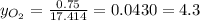 %
%



