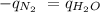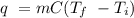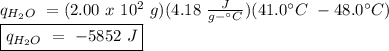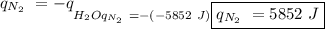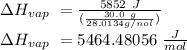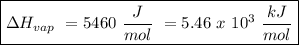Chemistry, 23.07.2019 02:20, bryanmcmillianjr
The heat of vaporization of a liquid (δhvap) is the energy required to vaporize 1.00 g of the liquid at its boiling point. in one experiment, 30.0 g of liquid nitrogen (boiling point = −196°c) is poured into a styrofoam cup containing 2.00 × 102 g of water at 48.1°c. calculate the molar heat of vaporization of liquid nitrogen if the final temperature of the water is 41.0°c.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, tashaunalewis4786
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1



Chemistry, 22.06.2019 17:00, marsjupiter2554
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
Do you know the correct answer?
The heat of vaporization of a liquid (δhvap) is the energy required to vaporize 1.00 g of the liquid...
Questions in other subjects:

Social Studies, 21.01.2021 01:00

Mathematics, 21.01.2021 01:00

Biology, 21.01.2021 01:00

Mathematics, 21.01.2021 01:00



Mathematics, 21.01.2021 01:00

History, 21.01.2021 01:00


Mathematics, 21.01.2021 01:00