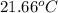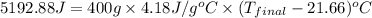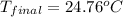Chemistry, 22.07.2019 23:20, autumnguidry7628
Aquantity of 2.00 × 102 ml of 0.461 m hcl is mixed with 2.00 × 102 ml of 0.231 m ba(oh)2 in a constant-pressure calorimeter of negligible heat capacity. the initial temperature of the hcl and ba(oh)2 solutions is the same at 21.66°c. for the process below, the heat of neutralization is −56.2 kj/mol. what is the final temperature of the mixed solutions? h+(aq) + oh−(aq) → h2o(l)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, NatalieKnows
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 23.06.2019 15:00, eazywalters
The coriolis effect influences neither wind speed nor wind direction wind speed both wind speed and wind direction wind direction
Answers: 1

Chemistry, 23.06.2019 16:00, Karinaccccc
The electron configuration for chromium is 1s22s22p63s23p63d54s1 instead of 1s22s22p63s23p63d44s1. the configuration is an exception to the pauli exclusion principle heisenberg uncertainty principle aufbau principle schrödinger equation
Answers: 3
Do you know the correct answer?
Aquantity of 2.00 × 102 ml of 0.461 m hcl is mixed with 2.00 × 102 ml of 0.231 m ba(oh)2 in a consta...
Questions in other subjects:




Mathematics, 28.08.2020 03:01

Mathematics, 28.08.2020 03:01

English, 28.08.2020 03:01


Business, 28.08.2020 03:01

Mathematics, 28.08.2020 03:01


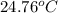
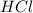 and
and 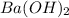 .
.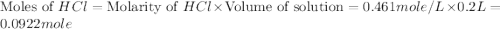
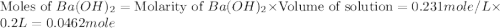
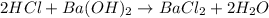
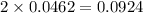 mole of HCl
mole of HCl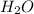
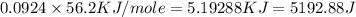
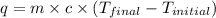
 = specific heat capacity =
= specific heat capacity = 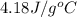
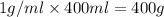
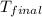 = final temperature = ?
= final temperature = ?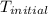 = initial temperature =
= initial temperature =