
Assume that each tablet's mass was 1,000 mg, and remember that you used 0,200 l of water each time,
compute the reaction rate to the nearest whole number using the formula below,
mass of tablet/volume of water
reaction rate = mas
reaction time
3°c reaction time = 138.5 sec
reaction rate = mg/l/sec
24°c reaction time = 34,2 sec
reaction rate = mg/l/sec
40°c reaction time = 26.3 sec
reaction rate = mg/l/sec
65°c reaction time = 14.2 sec
reaction rate = mg/l/sec


Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, coralstoner6793
Which of these will change if the air in aclosed bottle is heated? abcdthe mass of the airthe composition of the airthe air pressure in the bottlethe number of air molecules in the bottle
Answers: 3



Chemistry, 22.06.2019 13:30, xojade
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
Do you know the correct answer?
Assume that each tablet's mass was 1,000 mg, and remember that you used 0,200 l of water each time,<...
Questions in other subjects:






Arts, 21.07.2019 23:00

Arts, 21.07.2019 23:00

Arts, 21.07.2019 23:00

Arts, 21.07.2019 23:00

Arts, 21.07.2019 23:00


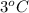 is, 36.1 mg/L/sec
is, 36.1 mg/L/sec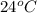 is, 146.2 mg/L/sec
is, 146.2 mg/L/sec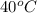 is, 190.1 mg/L/sec
is, 190.1 mg/L/sec is, 352.1 mg/L/sec
is, 352.1 mg/L/sec








