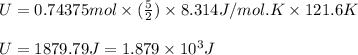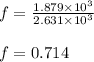Chemistry, 20.07.2019 02:30, aubriebv2020
Suppose 23.8 g of oxygen (o2) is heated at constant atmospheric pressure from 27.4°c to 149°c. (a) how many moles of oxygen are present? (take the molar mass of oxygen to be 32.0 g/mol) (b) how much energy is transferred to the oxygen as heat? (the molecules rotate but do not oscillate.) (c) what fraction of the heat is used to raise the internal energy of the oxygen?

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 23:30, Xavier8247
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
Do you know the correct answer?
Suppose 23.8 g of oxygen (o2) is heated at constant atmospheric pressure from 27.4°c to 149°c. (a) h...
Questions in other subjects:

Computers and Technology, 16.02.2021 06:10


Mathematics, 16.02.2021 06:10


Mathematics, 16.02.2021 06:10



English, 16.02.2021 06:10



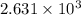
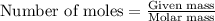
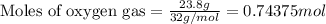
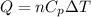
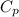 = specific heat capacity at constant pressure =
= specific heat capacity at constant pressure = 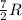 (For diatomic gas)
(For diatomic gas) = change in temperature =
= change in temperature = 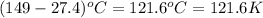
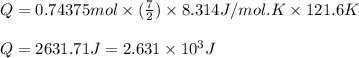
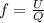
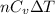
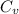 = specific heat capacity at constant pressure =
= specific heat capacity at constant pressure = 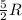 (For diatomic gas)
(For diatomic gas)