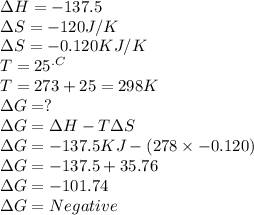Chemistry, 19.07.2019 22:20, haleybain6353
C2h4(g) + h2(g) → c2h6(g) δh = –137.5 kj; δs = –120.5 j/k calculate δg at 25 °c and determine whether the reaction is spontaneous. does δg become more negative or more positive as the temperature increases?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, pup88
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 20:30, huangjianhe135
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 23.06.2019 01:00, bsheepicornozj0gc
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Do you know the correct answer?
C2h4(g) + h2(g) → c2h6(g) δh = –137.5 kj; δs = –120.5 j/k calculate δg at 25 °c and determine wheth...
Questions in other subjects:






Mathematics, 15.04.2020 16:09

Mathematics, 15.04.2020 16:09


English, 15.04.2020 16:09

History, 15.04.2020 16:10