Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, david838843
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 07:30, isalih7256
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1


Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
Do you know the correct answer?
Calculate the change in entropy that occurs in the system when 4.20 mole of diethyl ether (\(\rm c_4...
Questions in other subjects:

English, 09.02.2021 16:00










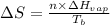
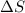 = entropy change of the system = ?
= entropy change of the system = ?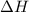 = enthalpy of vaporization = 34.6 kJ/mole
= enthalpy of vaporization = 34.6 kJ/mole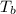 = normal boiling point =
= normal boiling point =