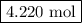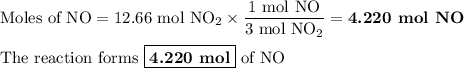Chemistry, 19.07.2019 21:30, makayyafreeman
According to the following balanced reaction, how many moles of no are formed from 12.66 moles of no2 if there is plenty of water present? 3 no2(g) + h2o(l) → 2 hno3(aq) + no(g

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 04:30, mamabates181981
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 08:30, itzhari101
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 10:00, youngchapo813p8d9u1
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3
Do you know the correct answer?
According to the following balanced reaction, how many moles of no are formed from 12.66 moles of no...
Questions in other subjects:




Social Studies, 02.10.2019 03:30

Mathematics, 02.10.2019 03:30

History, 02.10.2019 03:30


Social Studies, 02.10.2019 03:30