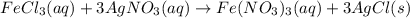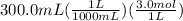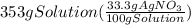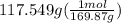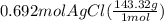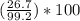Chemistry, 19.07.2019 20:10, laurielaparr2930
300. ml of a 3.0 m aqueous solution of iron (iii) chloride is mixed with 353 grams of a 33.3 mass % solution of silver (u) nitrate and water. 267 grams of a solid precipitate forms. what is the percent yield of the reaction assuming that the solubility of the solid precipitate in water is negligible.

Answers: 2
Similar questions

Chemistry, 02.08.2019 02:10, gregorio03
Answers: 1
Do you know the correct answer?
300. ml of a 3.0 m aqueous solution of iron (iii) chloride is mixed with 353 grams of a 33.3 mass %...
Questions in other subjects:


English, 13.08.2021 05:40


Mathematics, 13.08.2021 05:40




Mathematics, 13.08.2021 05:40

Mathematics, 13.08.2021 05:50

Mathematics, 13.08.2021 05:50