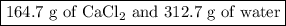Chemistry, 19.07.2019 05:20, kailahgranger
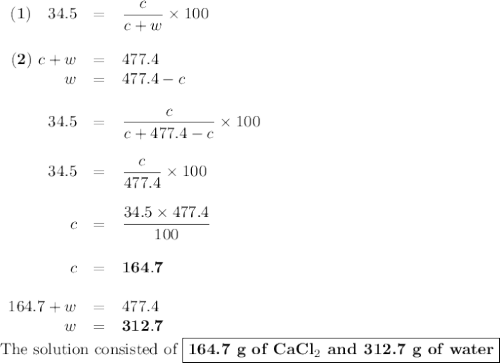
Asolution of cacl2 in water forms a mixture that is 34.5% calcium chloride by mass. if the total mass of the mixture is 477.4 g, what masses of cacl2 and water were used?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:20, anggar20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 10:00, zionlopez543
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 15:30, sanchez7489
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
Do you know the correct answer?
Asolution of cacl2 in water forms a mixture that is 34.5% calcium chloride by mass. if the total mas...
Questions in other subjects:

Mathematics, 08.07.2019 19:00




Mathematics, 08.07.2019 19:00




History, 08.07.2019 19:00

History, 08.07.2019 19:00