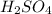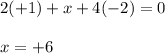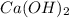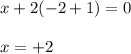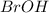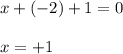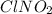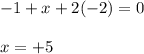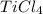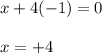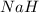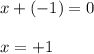Chemistry, 19.07.2019 02:10, Brightcord9679
Determine the oxidation states of the elements in the compounds listed. none of the oxygen-containing compounds are peroxides or superoxides. (a) h2so4 (b) ca(oh)2 (c) broh (d) clno2 (e) ticl4 (f) nah'

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:30, mimibear2932
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
Do you know the correct answer?
Determine the oxidation states of the elements in the compounds listed. none of the oxygen-containin...
Questions in other subjects:



English, 10.09.2021 06:40



Mathematics, 10.09.2021 06:40




History, 10.09.2021 06:40