
Chemistry, 13.07.2019 22:20, highschoolboy
The fraction of a radioactive isotope remaining at time t is (1/2)^t/t1/2 where t1/2 is the half-life. if the half-life of carbon−14 is 5,730 yr, what fraction of carbon−14 in a piece of charcoal remains after
(a) 14.0 yr?
(b) 1.900 × 10^4 yr? × 10 (enter your answer in scientific notation.)
(c) 1. × 10^5 yr? × 10 (enter your answer in scientific notation.)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, awdadaddda
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 03:30, krharris
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
Do you know the correct answer?
The fraction of a radioactive isotope remaining at time t is (1/2)^t/t1/2 where t1/2 is the half-lif...
Questions in other subjects:


Mathematics, 27.03.2020 19:33




Mathematics, 27.03.2020 19:45


Biology, 27.03.2020 19:45


Biology, 27.03.2020 19:45


 fraction of carbon−14 in a piece of charcoal remains after 14.0 years.
fraction of carbon−14 in a piece of charcoal remains after 14.0 years.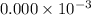 fraction of carbon−14 in a piece of charcoal remains after
fraction of carbon−14 in a piece of charcoal remains after 
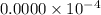 fraction of carbon−14 in a piece of charcoal remains after
fraction of carbon−14 in a piece of charcoal remains after 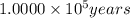 .
.![[A]=\frac{(\frac{1}{2})^t}{t_{\frac{1}{2}}}](/tpl/images/0086/3350/d6525.png)
![\log [A]=t\log[\frac{1}{2}]-\log [t_{\frac{1}{2}}]](/tpl/images/0086/3350/f81b0.png)
 = half life of the carbon−14 =5,730 years
= half life of the carbon−14 =5,730 years![\log [A]= 14 years\times (-3010)-\log [5,730 years]](/tpl/images/0086/3350/51128.png)
![[A]=1.065\times 10^{-8}](/tpl/images/0086/3350/2e677.png)
![\log [A]= 1.900\times 10^4 years\times (-3010)-\log [5,730 years]](/tpl/images/0086/3350/5f99c.png)
![[A]=0.000\times 10^{-3} [/tex](/tpl/images/0086/3350/ae3f2.png)
![\log [A]= 1.0000\times 10^5 years\times (-3010)-\log [5,730 years]](/tpl/images/0086/3350/a2485.png)
![[A]=0.0000\times 10^{-4}](/tpl/images/0086/3350/e1222.png)




