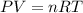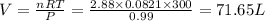Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid sodium azide (nan3). the other product is sodium metal. calculate the volume of nitrogen gas at 27 °c and 756 torr formed by the decomposition of 125 g of sodium azide.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:20, pandaman632
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 16:50, TheOriginal2x
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
Do you know the correct answer?
Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid so...
Questions in other subjects:




Biology, 20.11.2019 21:31


History, 20.11.2019 21:31


Biology, 20.11.2019 21:31

English, 20.11.2019 21:31


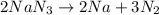
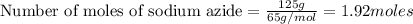
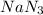 produce 3 moles of
produce 3 moles of 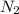
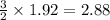 moles of
moles of