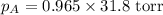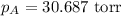Chemistry, 12.07.2019 18:10, damaricoleman42
Calculate the vapor pressure of a solution containing 24.6 g of glycerin (c3h8o3) in 134 ml of water at 30.0 ∘c. the vapor pressure of pure water at this temperature is 31.8 torr. assume that glycerin is not volatile and dissolves molecularly (i. e., it is not ionic) and use a density of 1.00 g/ml for the water.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, stellaglenn205
What reaction is taking place? 02 + c3h8 = h20 + co2
Answers: 1

Chemistry, 22.06.2019 06:30, Pizzapegasus1
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1
Do you know the correct answer?
Calculate the vapor pressure of a solution containing 24.6 g of glycerin (c3h8o3) in 134 ml of water...
Questions in other subjects:

History, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50

Biology, 17.03.2021 23:50


Mathematics, 17.03.2021 23:50

Computers and Technology, 17.03.2021 23:50



Mathematics, 17.03.2021 23:50


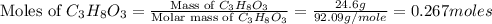
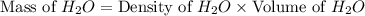
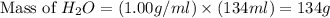
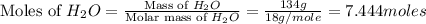
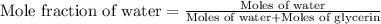
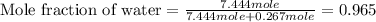
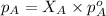
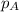 = vapor pressure of solution = ?
= vapor pressure of solution = ?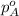 = vapor pressure of pure water= 31.8 torr
= vapor pressure of pure water= 31.8 torr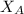 = mole fraction of water = 0.965
= mole fraction of water = 0.965