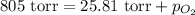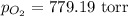Asample of oxygen gas was collected via water displacement. since the oxygen was collected via water displacement, the sample is saturated with water vapor. if the total pressure of the mixture at 26.4 °c is 805 torr, what is the partial pressure of oxygen? the vapor pressure of water at 26.4 °c is 25.81 mm hg.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, pennygillbert
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1

Chemistry, 21.06.2019 22:00, applereams
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
Do you know the correct answer?
Asample of oxygen gas was collected via water displacement. since the oxygen was collected via water...
Questions in other subjects:

Arts, 04.04.2020 00:04






Mathematics, 04.04.2020 00:04




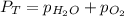
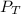 = total partial pressure = 805 torr
= total partial pressure = 805 torr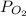 = partial pressure of oxygen gas = ?
= partial pressure of oxygen gas = ?
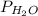 = partial pressure of water = 25.81 mm Hg = 25.81 torr
= partial pressure of water = 25.81 mm Hg = 25.81 torr