
Chemistry, 12.07.2019 02:20, Cupcake8189
Part 1. determine the molar mass of a 0.622-gram sample of gas having a volume of 2.4 l at 287 k and 0.850 atm. show your work.
part 2. if this sample was placed under extremely low temperature, describe how the actual volume would compare to the predicted volume. explain your answer.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 22:30, medinajocelyn45
Which compound most likely has the greatest bond energy?
Answers: 2
Do you know the correct answer?
Part 1. determine the molar mass of a 0.622-gram sample of gas having a volume of 2.4 l at 287 k and...
Questions in other subjects:


History, 10.11.2020 09:20

English, 10.11.2020 09:20


History, 10.11.2020 09:20

Mathematics, 10.11.2020 09:20

Social Studies, 10.11.2020 09:20

History, 10.11.2020 09:20

Computers and Technology, 10.11.2020 09:20

Social Studies, 10.11.2020 09:20


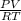
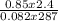
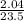 = 0.087mole
= 0.087mole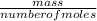
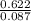 =7.15gmol⁻¹
=7.15gmol⁻¹



