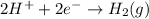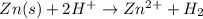Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2


Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 23:30, bxymichelle
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3
Do you know the correct answer?
Consider the chemical reaction below.
zn(s)+ 2h*(aq) → zn2+ (aq) + h2(g)
which half reac...
zn(s)+ 2h*(aq) → zn2+ (aq) + h2(g)
which half reac...
Questions in other subjects:






Mathematics, 15.07.2019 12:00


Computers and Technology, 15.07.2019 12:00


Mathematics, 15.07.2019 12:00