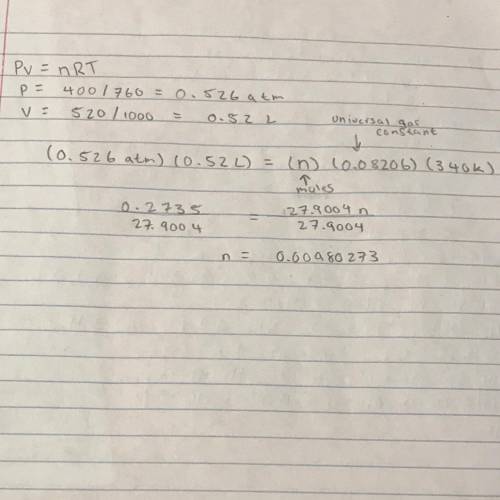Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, btcastongia
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3



Chemistry, 22.06.2019 14:00, claudia122752
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
Do you know the correct answer?
How many moles of gas does it take to occupy 520 ml at a pressure of 400 torr and a temperature of 3...
Questions in other subjects:

History, 15.12.2020 19:30


Business, 15.12.2020 19:30


English, 15.12.2020 19:30



Mathematics, 15.12.2020 19:30

Physics, 15.12.2020 19:30