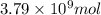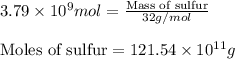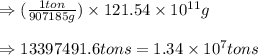Be sure to answer all parts. the annual production of sulfur dioxide from burning coal and fossil fuels, auto exhaust, and other sources is about 26 million tons. the equation for the reaction is s(s) + o2(g) → so2(g) if 2.68 × 107 tons of sulfur dioxide formed, how many tons of sulfur were present in the original materials? assume 100% yield. × 10 tons enter your answer in scientific notation.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 19:40, trodgers0202
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests. which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3
Do you know the correct answer?
Be sure to answer all parts. the annual production of sulfur dioxide from burning coal and fossil fu...
Questions in other subjects:



Physics, 27.08.2019 07:00

Computers and Technology, 27.08.2019 07:00


Mathematics, 27.08.2019 07:00


History, 27.08.2019 07:00

Chemistry, 27.08.2019 07:00


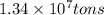
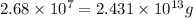
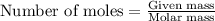 ......(2)
......(2)
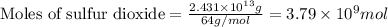

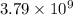 moles of sulfur dioxide will be produced from =
moles of sulfur dioxide will be produced from = 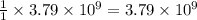 moles of sulfur.
moles of sulfur.