
Chemistry, 08.07.2019 23:40, sairaanwar67
For the reaction h2(g) + co2(g) ⇌ h2o(g) + co(g) at 700ºc, kc = 0.534. calculate the number of moles of h2 that are present at equilibrium if a mixture of 0.300 mole of co and 0.300 mole of h2o is heated to 700ºc in a 10.0-l container.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:10, lindasuebairdoyjpf7
How many grams of naoh are needed to make 0.250 liter of a 0.500 m solution of naoh? 0.125 g 5.00 g 2.00 g
Answers: 1

Chemistry, 21.06.2019 22:50, ajaydonlee
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2


Chemistry, 23.06.2019 02:00, bagofmud8339
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
Do you know the correct answer?
For the reaction h2(g) + co2(g) ⇌ h2o(g) + co(g) at 700ºc, kc = 0.534. calculate the number of moles...
Questions in other subjects:

Mathematics, 19.05.2021 09:20

Mathematics, 19.05.2021 09:20


Chemistry, 19.05.2021 09:20



English, 19.05.2021 09:20


Mathematics, 19.05.2021 09:20


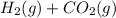 ⇄
⇄ 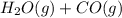 at 700ºC.
at 700ºC.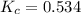
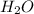 is heated to 700ºC.
is heated to 700ºC.![[H_2O]=\frac{300 mol}{10.0L}=30 M](/tpl/images/0067/3669/12641.png)
![H_2O=[H_2O]=\frac{300 mol}{10.0L}=30 M](/tpl/images/0067/3669/82ae5.png)
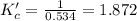
![K_c'=\frac{[H_2][CO_2]}{[CO][H_2O]}](/tpl/images/0067/3669/df550.png)
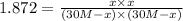
![[H_2]](/tpl/images/0067/3669/08a38.png)
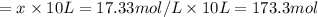
![[H_2]_{eq}=0.0173M](/tpl/images/0067/3669/87baf.png)
![Kc=\frac{[H_2O][CO]}{[H_2][CO_2]}](/tpl/images/0067/3669/4d854.png)
![[CO_2]_0=[H_2]_0=\frac{0.300mol}{10.0L} =0.030M](/tpl/images/0067/3669/89e63.png)
 due to the equilibrium, the law of mass action takes the following form:
due to the equilibrium, the law of mass action takes the following form: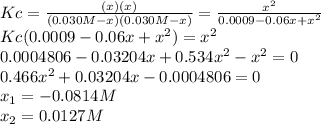
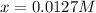
![[H_2]_{eq}=0.030M-0.0127M=0.0173M](/tpl/images/0067/3669/92f4c.png)




