
Be sure to answer all parts. nitric oxide (no) reacts with oxygen gas to form nitrogen dioxide (no2), a dark brown gas: 2no(g) + o2(g) → 2no2(g)
in one experiment, 0.857 mol of no is mixed with 0.498 mol of o2.
determine which of the two reactants is the limiting reactant. calculate also the number of moles of no2 produced. limiting reactant: moles of no2 produced: moles

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2

Chemistry, 22.06.2019 15:20, merrickrittany
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
Do you know the correct answer?
Be sure to answer all parts. nitric oxide (no) reacts with oxygen gas to form nitrogen dioxide (no2)...
Questions in other subjects:

History, 19.12.2019 00:31

Biology, 19.12.2019 00:31

English, 19.12.2019 00:31


English, 19.12.2019 00:31

Mathematics, 19.12.2019 00:31

English, 19.12.2019 00:31


Health, 19.12.2019 00:31

Social Studies, 19.12.2019 00:31


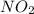 will be produced.
will be produced.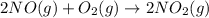
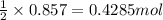 of
of 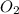
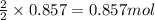 of
of 



