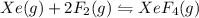Chemistry, 08.07.2019 18:10, alemorachis49
1) consider the following reaction at equilibrium. what effect will reducing the pressure of the reaction mixture have on the system? xe(g) + 2 f2(g) ? xef4(g) a)the equilibrium constant will decrease. b)no effect will be observed. c)the reaction will shift to the right in the direction of products. d) the equilibrium constant will increase e) the reaction will shift to the left in the direction of reactants.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, KnMcdonaldk93906
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1


Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Do you know the correct answer?
1) consider the following reaction at equilibrium. what effect will reducing the pressure of the rea...
Questions in other subjects:

Mathematics, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

English, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

English, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

English, 12.09.2020 01:01