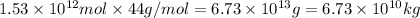Calcium oxide or quicklime (cao) is used in steelmaking, cement manufacture, and pollution control. it is prepared by the thermal decomposition of calcium carbonate: caco3(s) → cao(s) co2(g) calculate the yearly release of co2 (in kg) to the atmosphere if the annual production of cao in the united states is 8.6 × 1010 kg.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 08:30, waterborn7152
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1
Do you know the correct answer?
Calcium oxide or quicklime (cao) is used in steelmaking, cement manufacture, and pollution control....
Questions in other subjects:

Mathematics, 19.09.2019 07:30

Mathematics, 19.09.2019 07:30









 into the atmosphere is
into the atmosphere is  .
.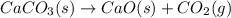


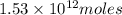 of CaO moles of carbon-dioxide moles produced will be:
of CaO moles of carbon-dioxide moles produced will be: of carbon-dioxide
of carbon-dioxide moles of carbon-dioxide:
moles of carbon-dioxide: