
Chemistry, 05.02.2020 12:00, hillisaiah734
The reform reaction between steam and gaseous methane (ch4) produces "synthesis gas," a mixture of carbon monoxide gas and dihydrogen gas. synthesis gas is one of the most widely used industrial chemicals, and is the major industrial source of hydrogen.
suppose a chemical engineer studying a new catalyst for the reform reaction finds that 924. liters per second of methane are consumed when the reaction is run at 261.°c and 0.96atm. calculate the rate at which dihydrogen is being produced. give your answer in kilograms per second. round your answer to 2 significant digits.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 15:30, alaf05160
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks. energy was destroyed inside the blocks. energy was absorbed into the blocks from outside the system. energy was transferred from the warmer block to the cooler block.
Answers: 2

Do you know the correct answer?
The reform reaction between steam and gaseous methane (ch4) produces "synthesis gas," a mixture of c...
Questions in other subjects:






Mathematics, 17.09.2019 02:00


Mathematics, 17.09.2019 02:00

Mathematics, 17.09.2019 02:00

History, 17.09.2019 02:00


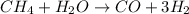 Haber reaction
Haber reaction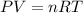
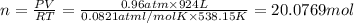
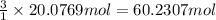 of dihydrogen
of dihydrogen




