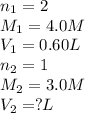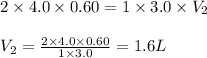Chemistry, 07.07.2019 11:10, silveriomanzuet
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4?
h2so4 + naoh → na2so4 + h2o
1.2 l
1.6 l
2.4 l
2.8 l

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, nadikadiaz1
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1

Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 06:20, ratpizza
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1

Chemistry, 23.06.2019 11:20, mjwenz8018
Ajar is tightly sealed at 22°c and 772 torr what is the pressure inside a jar after its been heated to 178°c
Answers: 1
Do you know the correct answer?
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4?
h2so4 + na...
h2so4 + na...
Questions in other subjects:

Health, 16.09.2021 01:00

English, 16.09.2021 01:00

History, 16.09.2021 01:00

Mathematics, 16.09.2021 01:00

Business, 16.09.2021 01:00

Physics, 16.09.2021 01:00

English, 16.09.2021 01:00


Law, 16.09.2021 01:00

Mathematics, 16.09.2021 01:00


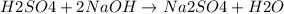
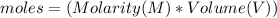
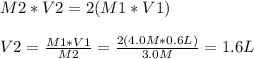
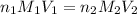
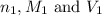 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 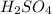
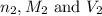 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.