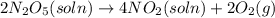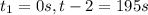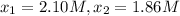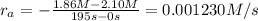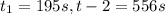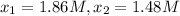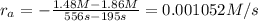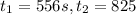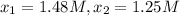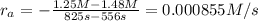The decomposition of n2o5 can be described by the equation.
2n2o5 (soln) > 4no2 (soln) + 2...

Chemistry, 07.07.2019 03:10, natalie2sheffield
The decomposition of n2o5 can be described by the equation.
2n2o5 (soln) > 4no2 (soln) + 2 (g)
given this data for the reaction at 45 degrees c in carbon tetrachloride solution, calculate the average rate for each successive time interval.
t(s) [n2o5] (m)
0 2.10
195 1.86
556 1.48
825 1.25
interval: 0 s to 195 s
reaction rate= /s
195 s to 556 s
reaction rate= /s
556 s to 825 s
reaction rate= /s

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 23.06.2019 03:30, elijahjacksonrp6z2o7
In general metals get as you move from left to right across the periodic table.
Answers: 1

Chemistry, 23.06.2019 05:40, MyChannelBruh6896
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 15.04.2021 21:20





Mathematics, 15.04.2021 21:20

Computers and Technology, 15.04.2021 21:20




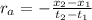
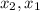 : concentration at time
: concentration at time 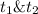 respectively.
respectively.