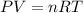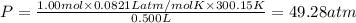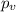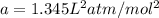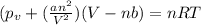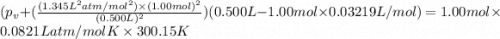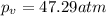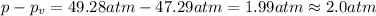Chemistry, 06.07.2019 05:10, ramberson101
If 1.00 mol of argon is placed in a 0.500-l container at 27.0 degree c , what is the difference between the ideal pressure (as predicted by the ideal gas law) and the real pressure (as predicted by the van der waals equation)? for argon, a=1.345(l2⋅atm)/mol2 and b=0.03219l/mol. express your answer to two significant figures and include the appropriate units.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 02:30, sotoamerica0814
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3
Do you know the correct answer?
If 1.00 mol of argon is placed in a 0.500-l container at 27.0 degree c , what is the difference betw...
Questions in other subjects:






Mathematics, 15.12.2020 20:30

Health, 15.12.2020 20:30

Mathematics, 15.12.2020 20:30

Biology, 15.12.2020 20:30

Mathematics, 15.12.2020 20:30