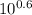Chemistry, 30.01.2020 02:03, lewisf2578
Calculation of original ph from final ph after titration a biochemist has 100 ml of a 0.10 m solution of a weak acid with a pka of 6.3. she adds 6.0 ml of 1.0 m hcl, which changes the ph to 5.7. what was the ph of the original solution?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:00, alaina3792
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 03:30, jabper5522
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1
Do you know the correct answer?
Calculation of original ph from final ph after titration a biochemist has 100 ml of a 0.10 m solutio...
Questions in other subjects:




English, 01.08.2019 18:30