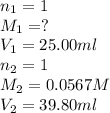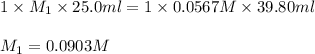Chemistry, 06.07.2019 00:20, cocothunder635
Formic acid (hco2h, ka = 1.8 × 10-4) is the principal component in the venom of stinging ants. what is the molarity of a formic acid solution if 25.00 ml of the formic acid solution requires 39.80 ml of 0.0567 m naoh to reach the equivalence point?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 22:30, arodavoarodavo
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 01:30, bgarrison364
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1
Do you know the correct answer?
Formic acid (hco2h, ka = 1.8 × 10-4) is the principal component in the venom of stinging ants. what...
Questions in other subjects:











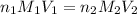
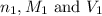 are the n-factor, molarity and volume of formic acid which is
are the n-factor, molarity and volume of formic acid which is 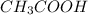
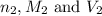 are the n-factor, molarity and volume of sodium hydroxide base which is NaOH.
are the n-factor, molarity and volume of sodium hydroxide base which is NaOH.