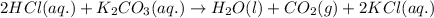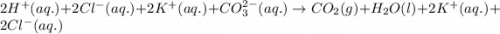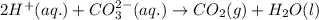Chemistry, 05.07.2019 22:10, chloeholt123
Enter the balanced complete ionic equation for hcl(aq)+k2co3(aq)→h2o(l)+co2(g)+kcl (aq). express your answer as a chemical equation. identify all of the phases in your answer.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 03:30, tbeck225
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 23.06.2019 09:00, ashhull2002
Need ! assume that the variables x and y are directly related. if k = 8, what is the value for each of the following points? be sure and record your data to be used in the following problem. x y k 0.
Answers: 2

Do you know the correct answer?
Enter the balanced complete ionic equation for hcl(aq)+k2co3(aq)→h2o(l)+co2(g)+kcl (aq). express you...
Questions in other subjects:

Mathematics, 15.07.2019 03:30


Mathematics, 15.07.2019 03:30


Mathematics, 15.07.2019 03:30

Mathematics, 15.07.2019 03:30



Mathematics, 15.07.2019 03:30