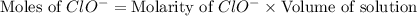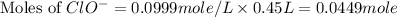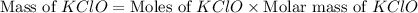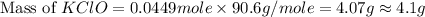Chemistry, 05.07.2019 20:20, TheJanko4526
What mass of potassium hypochlorite (fw-90.6 g/mol) must be added to 4.50 x 10 ml of water to give a solution with ph 10.20? [ka(hcio) 4.0 x 10-8] 0.032g ? 2.4 g 04.1 g 9.1 g 20. g

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, 19thomasar
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2

Chemistry, 22.06.2019 15:30, ricardotavarez6
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 16:10, nauticatyson9
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1
Do you know the correct answer?
What mass of potassium hypochlorite (fw-90.6 g/mol) must be added to 4.50 x 10 ml of water to give a...
Questions in other subjects:



Mathematics, 28.04.2021 01:40



Geography, 28.04.2021 01:40

Mathematics, 28.04.2021 01:40


Mathematics, 28.04.2021 01:40


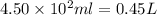
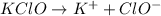
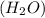 to give,
to give,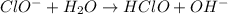
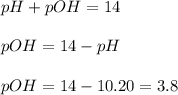
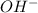 concentration.
concentration.![pOH=-\log [OH^-]](/tpl/images/0055/2981/1fac1.png)
![3.8=-\log [OH^-]](/tpl/images/0055/2981/a714c.png)
![[OH^-]=1.58\times 10^{-4}M](/tpl/images/0055/2981/110da.png)
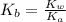
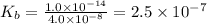
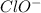 .
.![K_b=\frac{[OH^-][HClO]}{[ClO^-]}](/tpl/images/0055/2981/9b5c8.png)
![[OH^-]=[HClO]=1.58\times 10^{-4}M](/tpl/images/0055/2981/ec220.png)
![2.5\times 10^{-7}=\frac{(1.58\times 10^{-4})^2}{[ClO^-]}](/tpl/images/0055/2981/2ef50.png)
![[ClO^-]=0.0999M](/tpl/images/0055/2981/776df.png)