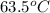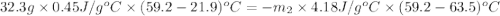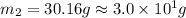Chemistry, 05.07.2019 19:10, stinematesa
A32.2 g iron rod, initially at 21.9 c, is submerged into an unknown mass of water at 63.5 c. in an insulated container. the final temperature of the mixture upon reaching thermal equilibrium is 59 2 c what is the mass of the water? express your answer to two significant figures

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, rosieposie27
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1


Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
Do you know the correct answer?
A32.2 g iron rod, initially at 21.9 c, is submerged into an unknown mass of water at 63.5 c. in an i...
Questions in other subjects:

Mathematics, 11.10.2021 09:30


Mathematics, 11.10.2021 09:30


Mathematics, 11.10.2021 09:30




History, 11.10.2021 09:30

Mathematics, 11.10.2021 09:30


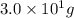
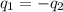
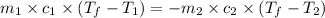
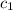 = specific heat of iron metal =
= specific heat of iron metal = 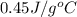
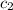 = specific heat of water =
= specific heat of water = 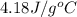
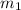 = mass of iron metal = 32.3 g
= mass of iron metal = 32.3 g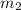 = mass of water = ?
= mass of water = ?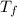 = final temperature of mixture =
= final temperature of mixture = 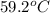
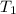 = initial temperature of iron metal =
= initial temperature of iron metal = 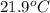
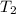 = initial temperature of water =
= initial temperature of water =