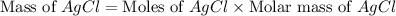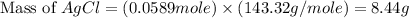Chemistry, 05.07.2019 19:10, katelynbychurch
When silver nitrate reacts with barium chloride, silver chloride and barium nitrate are formed. how many grams of silver chloride are formed when 10.0 g of silver nitrate reacts with 15.0 g of barium chloride? when silver nitrate reacts with barium chloride, silver chloride and barium nitrate are formed. how many grams of silver chloride are formed when 10.0 g of silver nitrate reacts with 15.0 g of barium chloride? 18.8 g 8.44 g 11.9 g 9.40 g

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, jivsf
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 23.06.2019 12:30, 1Angel2Got3Brains
Is the genie in the bottle experiment a physical or chemical change/reaction?
Answers: 1

Chemistry, 23.06.2019 12:50, jasonoliva13
How many energy levels contain electrons in an atom of zirconium (zr)?
Answers: 1

Chemistry, 23.06.2019 13:30, lashayreed02
What would happen if no were added to n(g)+o2=2no(g) at equilibrium?
Answers: 1
Do you know the correct answer?
When silver nitrate reacts with barium chloride, silver chloride and barium nitrate are formed. how...
Questions in other subjects:

Mathematics, 03.02.2021 19:50

Mathematics, 03.02.2021 19:50

Mathematics, 03.02.2021 19:50



Chemistry, 03.02.2021 19:50



English, 03.02.2021 19:50


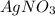 = 10.0 g
= 10.0 g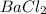 = 15.0 g
= 15.0 g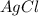 = 143.32 g/mole
= 143.32 g/mole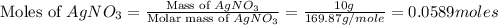
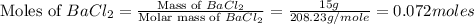
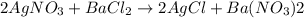
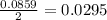 moles of
moles of